Preventing Chemical Accidents in the Clinical Lab
How to limit and contain lab accidents involving hazardous chemicals

Clinical laboratory staff often find themselves handling chemicals with varying levels of risk. In a fast-paced clinical laboratory, it can sometimes be difficult to keep track of these risks, along with proper handling, storage, and disposal of each of these hazardous chemicals. However, improper handling and storage of chemicals can result in chemical accidents that can lead to serious injury and even death.
This article will explore two potential chemical accidents involving two of the most common chemicals found in clinical laboratories to illustrate valuable safety guidelines. I will review the dangers of each chemical, what to do in each scenario, and how to avoid similar scenarios in the future.
Scenario one: Xylene vs water
One of your lab mates is performing a protocol that involves the use of xylene. She goes to the fume hood, opens the bottle of xylene, dispenses 100 ml of xylene into a glass beaker, then takes the beaker back to her bench, which is next to the sink. Another lab mate goes to the sink to wash their hands, some water from the sink splashes into the beaker of xylene, and suddenly, the beaker full of xylene erupts into flames. What should you do?
When xylene meets water, it releases flammable gases that are so volatile that they can ignite spontaneously. Xylene is also dangerous via inhalation, causing nose and throat irritation, and prolonged exposure can cause harm to the nervous system.
The first thing to do in this scenario is to extinguish the fire and evaluate the employee for injuries. Do not use water to extinguish this fire! Water will not be effective and may react further with the xylene. The proper fire extinguisher for this type of fire is an ABC dry chemical unit that can be used to extinguish Class A, B, and C fires.

Once the fire is extinguished, evaluate your staff member for burns. If they were burned, place the burned skin under cold, running water for at least 30 minutes. If severe burns are present, evidenced by blistering skin, seek immediate medical assistance for this employee.
To avoid this from happening again, always use xylene in a fume hood. In this first scenario, the technician initially does the right thing by using the xylene in the fume hood, but her mistake was then removing the beaker of xylene from the fume hood. This not only exposed the other staff member to dangerous xylene fumes, but also risked exposing the xylene to water. Xylene should always be kept away from sources of water.
Another precaution for protecting staff from dangerous chemical explosions when xylene is exposed to water is to install plastic shields next to every lab sink to prevent water from splashing onto other work surfaces. These plastic shields can also help prevent samples from becoming contaminated with city water from the sink.
When handling xylene, laboratory staff should wear proper personal protective equipment (PPE), including lab coats, goggles, gloves, and face shields.
Xylene waste should be disposed of as hazardous waste, per your institution’s policies.
Scenario two: Hydrochloric acid vs sodium hydroxide
After performing a protocol, you have some sodium hydroxide waste that requires proper disposal. You go to the chemical fume hood to pour your sodium hydroxide waste into the sodium hydroxide waste container in the lab. The lab keeps large amounts of both chemical containers and waste containers in the fume hood for easy access. You reach for the sodium hydroxide waste container but accidentally grab the container right next to it, which contains concentrated hydrochloric acid. You proceed to pour your sodium hydroxide waste into the bottle of hydrochloric acid, when suddenly, a violent reaction occurs and the chemicals boil and spill out of the container onto your arm.
When strong acids (hydrochloric acid) are combined with strong bases (sodium hydroxide), a violent, heat-releasing reaction occurs, causing rapid and aggressive boiling of the combined chemicals, which can boil out of their container, damaging equipment and causing serious chemicals burns to lab staff.
In this second scenario, your priority is to get help for your chemical burn. Quickly remove all contaminated clothing. Wash the exposed skin with large amounts of water for at least 30 minutes and then seek medical attention.
The contaminated clothing should be disposed of as hazardous waste. After tending to injuries, contain the spill using a chemical spill kit and communicate with Environmental Health & Safety (EH&S) to receive immediate instructions as to whether the lab should evacuate and allow EH&S staff to clean the spill, or if your lab should clean the spill using its own chemical spill kit.
IMPORTANT NOTE: Chemical spill kits do expire, so please check your kit annually. All lab staff should be regularly reminded of the location of the lab’s spill kits, and training on the proper use of the spill kit should be conducted with each new member who joins the lab, in addition to annual training for all lab staff to ensure knowledge is retained over time.
To avoid this scenario in the future, waste accumulation areas should always be isolated from chemical storage areas. In addition, chemicals need to be segregated not only in the chemical storage area, but also in the chemical waste storage area of the lab. Proper segregation prevents chemicals that react strongly when combined from being stored together to avoid lab accidents.
Proper chemical segregation in a laboratory is illustrated in the following infographic:
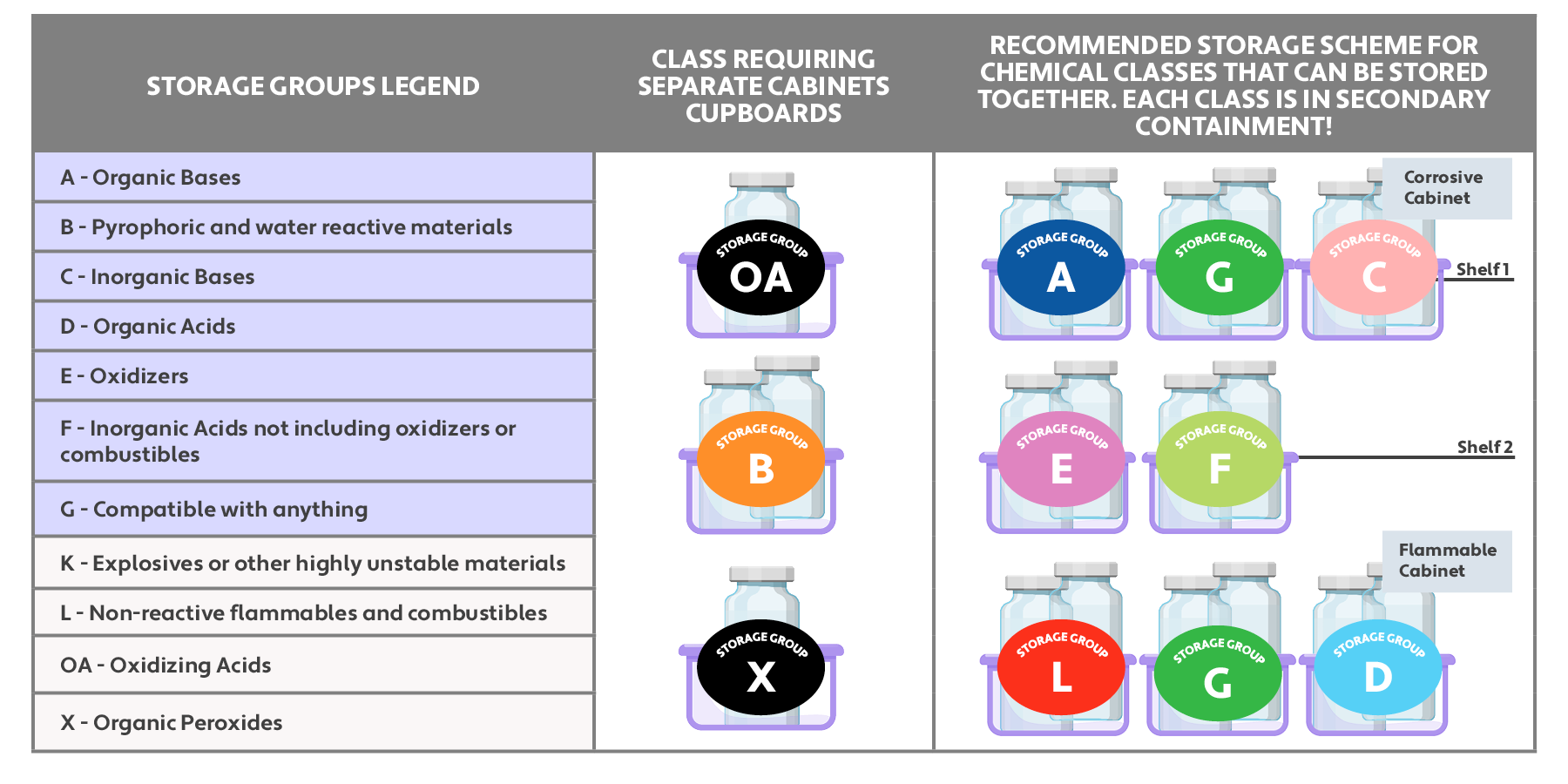
Lab staff should also wear proper PPE when handling strong acids and bases, including lab coats, goggles, gloves, and face shields.
Strong acids and bases should always be disposed of as hazardous waste.

